- Your cart is empty
- Continue Shopping
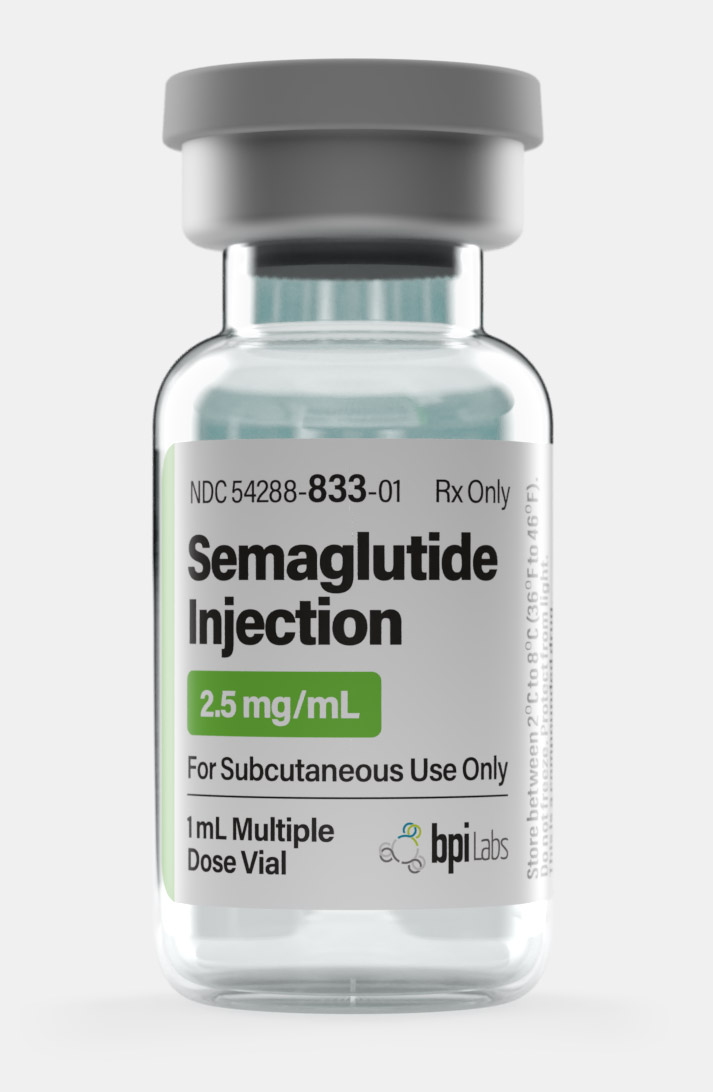
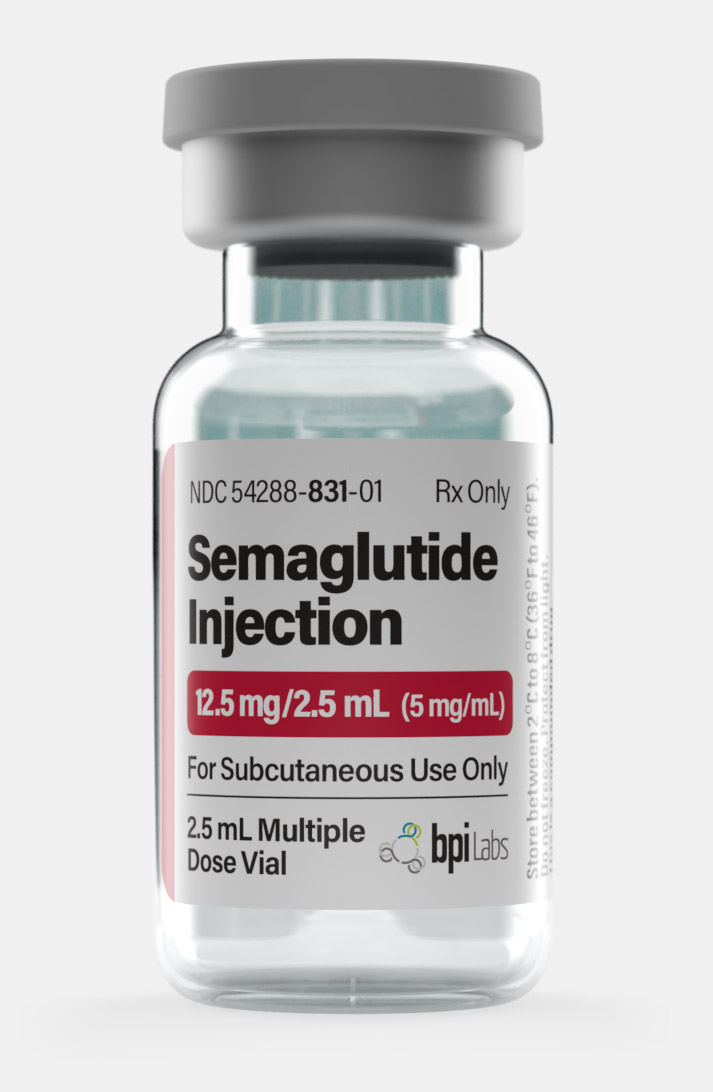
Buy Semaglutide Weight Loss Injection
Semaglutide is used for weight loss, to lower blood sugar levels in type 2 diabetes, and to reduce the risk of major cardiovascular events and chronic kidney disease in certain adult patients.
Semaglutide is a GLP-1 agonist that works by reducing appetite, delaying gastric emptying, increasing insulin release, and lowering the amount of glucagon released.
Semaglutide brand names are Ozempic, Rybelus, and Wegovy , which are made by Novo Nordisk, and are FDA-approved for different conditions. Semaglutide FDA approval was first granted on December 5, 2017, for the brand Ozempic.
What is semaglutide used for?
- Semaglutide is used for weight loss and weight maintenance in patients 12 years and older with obesity
- Used for reducing major cardiovascular event risks (such as heart attack, stroke, or death) in adults with type 2 diabetes with known heart disease
- Injection is given 1 time a week under the skin of the stomach (belly), the thigh, or the upper arm, using an autoinjector
- This injection should be used in addition to a reduced-calorie diet and increased physical activity.
How does semaglutide work?
Semaglutide’s mechanism of action involves mimicking a natural hormone in the body called GLP-1 which:
- Stimulates insulin production from the pancreas
- Reduces liver sugar production
- Slows down digestion
- Helps control appetite and food intake.
GLP-1 is a naturally occurring hormone that is released when we eat, to help us regulate our food intake. Semaglutide works by activating GLP-1 receptors, this lowers appetite, slows how quickly the stomach empties, and increases insulin production, so you feel fuller for longer and eat less, which leads to weight loss, control of blood sugar levels, and reduces the risk of cardiovascular disease.
The way semaglutide works for kidney-related risk reduction is not fully understood.
Semaglutide side effects
Common semaglutide side effects
Common semaglutide side effects may include low blood sugar (in people with type 2 diabetes), upset stomach, heartburn, burping, gas, bloating, nausea, vomiting, stomach pain, loss of appetite, diarrhea, constipation, stomach flu symptoms, headache, dizziness, tiredness.
Stomach or gastrointestinal side effects are common, but they tend to be mild and clear up in a few weeks and usually will not interfere with long-term treatment. Stomach side effects can be more common with higher doses.
Serious semaglutide side effects
Get emergency medical help if you have signs of an allergic reaction: hives, itching, dizziness, fast heartbeats, difficulty breathing, swelling of your face, lips, tongue, or throat.
Call your doctor at once if you have:
- vision changes;
- unusual mood changes, thoughts about hurting yourself;
- pounding heartbeats or fluttering in your chest;
- a light-headed feeling, like you might pass out;
- signs of a thyroid tumor – swelling or a lump in your neck, trouble swallowing, a hoarse voice, feeling short of breath;
- symptoms of pancreatitis – severe pain in your upper stomach spreading to your back, nausea with or without vomiting, fast heart rate;
- gallbladder problems – upper stomach pain, fever, clay-colored stools, jaundice (yellowing of the skin or eyes);
- low blood sugar – headache, hunger, weakness, sweating, confusion, irritability, dizziness, fast heart rate, or feeling jittery;
- kidney problems – swelling, urinating less, feeling tired or short of breath; or
- stomach flu symptoms – stomach cramps, vomiting, loss of appetite, diarrhea (may be watery or bloody).
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Additional Information
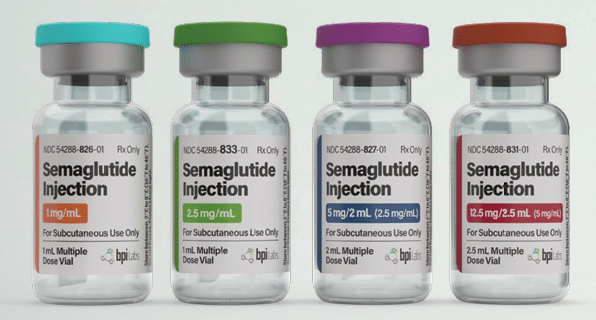
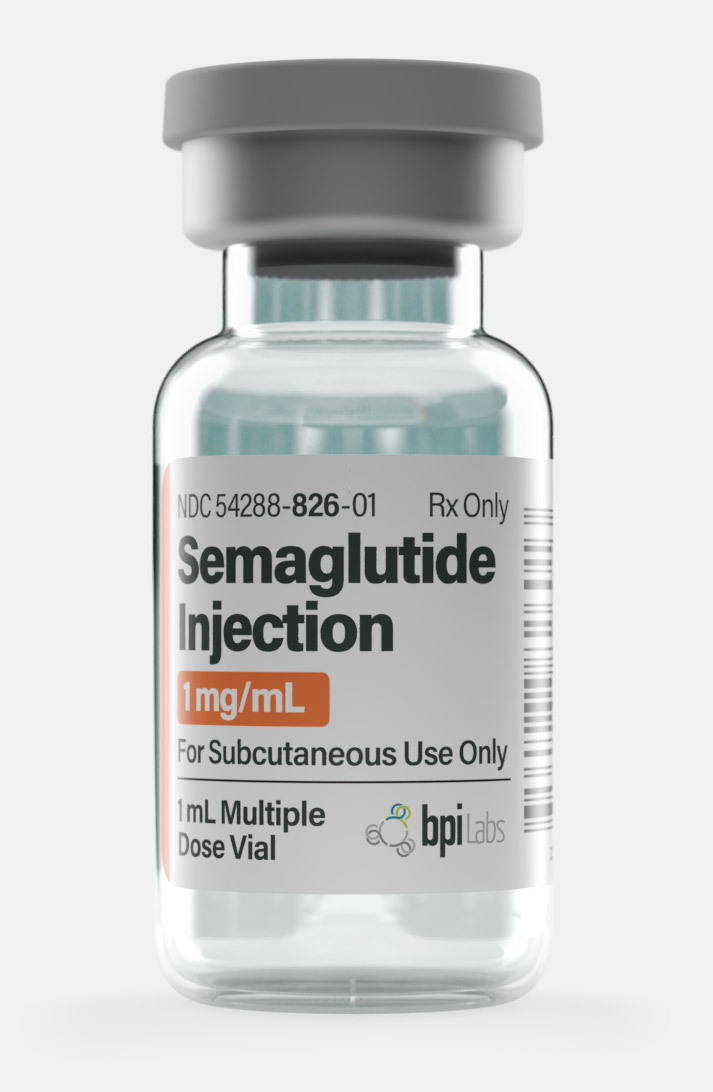
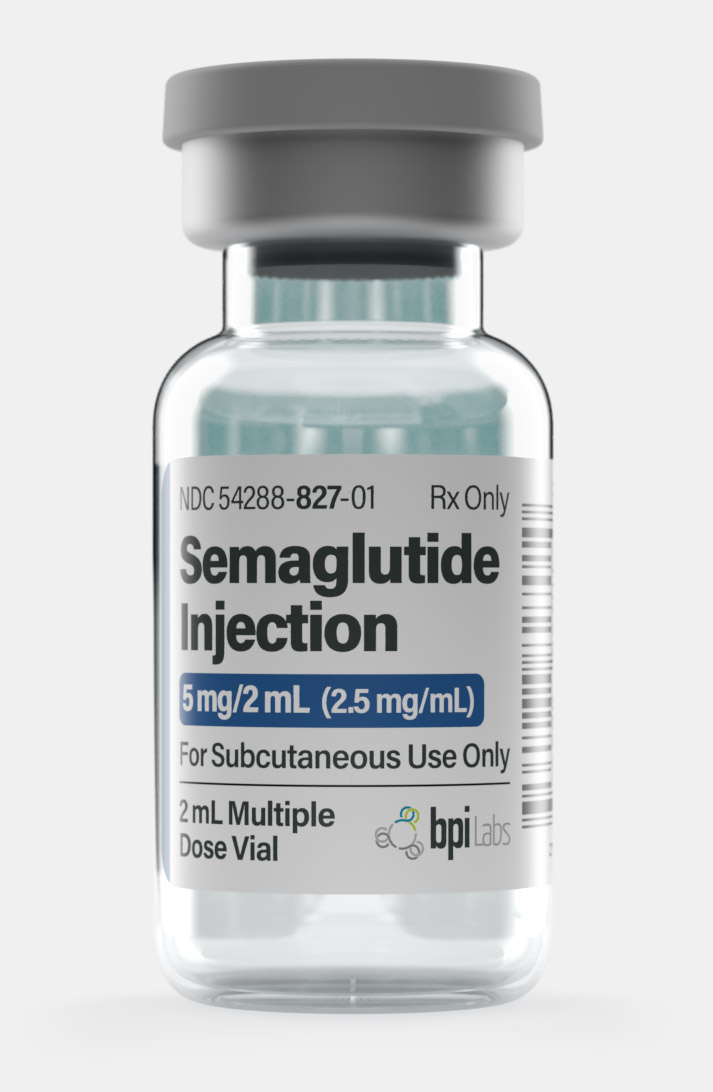
| Quantity | 12.5mg – Monthly, 1mg – Monthly, 2.5mg – Monthly, 5mg – Monthly |
|---|





Reviews
There are no reviews yet.